Zogenix's Long-Awaited Drug Approved With a Warning
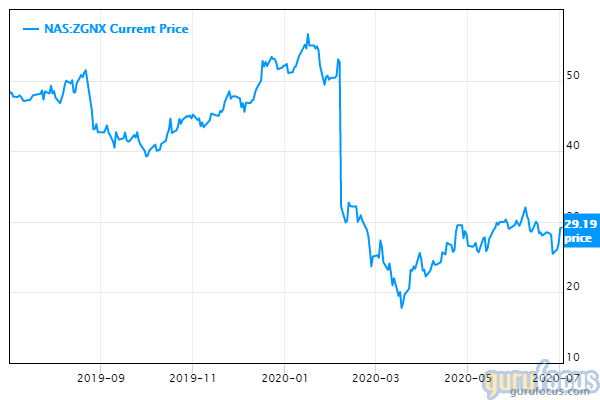
It's been a bumpy ride for Zogenix Inc. (NASDAQ:ZGNX) shareholders over the past three years, but at just more than $29, the stock is well below its average 12-month price target of $44.60.
The Emeryville, California-based biotech develops and markets therapies for people with serious and life-threatening rare central nervous system disorders and medical conditions. It currently has one product, Fintepla, which is used to treat seizures in children two years and older with Dravet syndrome, a rare, pediatric-onset form of epilepsy.
The company's shares dropped more than 9% on June 26, the day after Fintepla's was approved. Bio World reported that SVB Leerink's Marc Goodman wrote Thursday that "the FDA delayed approval two previous times, there has been investor skepticism regarding this approval, so we would expect the stock to move nicely tomorrow into the $35-40 range." That didn't happen.
Investors were likely wary that Fintepla was approved under the condition that its package insert carry a black box warning. This warning is required by the Food and Drug Administration for certain medications that have serious safety issues. This warning often greatly limits use of a medication and can be the death knell for others.
In the case of Fintepla, the warning notes that the treatment is associated with valvular heart disease and pulmonary hypertension. As a result, patients have to have echocardiograms before treatment, every six months after and once every three to six months once they stop using the drug.
Despite this caveat, Leerink's Goodman estimated in March that Fintepla would score U.S. sales of about $22 million this year and more than four times that in 2021. There are an estimated 6,000 to 8,000 Dravet syndrome patients in the U.S. The lead investigator of the drug's study, Kelly Knupp, M.D., of Children's Hospital Colorado, said Fintepla has the potential to be an important new treatment option for these patients, according to FierceBiotech.
Zogenix is also testing the drug for a rare and severe form of childhood-onset epilepsy and for a deficiency that causes progressive and severe muscle weakness that inhibits movement, breathing, eating/nutrition and other normal functions.
Zogenix was founded in 2006. It previously went under the name SJ2 Therapeutics Inc. The company has a market cap of about $1.6 billion. First-quarter sales were $1.2 million; the company had no sales in the first three months of 2019. Net loss for the first quarter was $25.8 million, or 54 cents per share, compared to a net loss of $35.2 million, or 83 cents per share, in the first quarter ended March 31, 2019
Out of five analysts, two rate the company a buy and three give it a strong buy, according to Yahoo Finance.
Disclosure: The author has no position in Zogenix.
Read more here:
New Pharma ETF Focuses on Testing and Treatment for Infectious Diseases--but Not Covid-19
Invitae's Market Opportunity Grows to $45 Billion After Latest Acquisition
Not a Premium Member of GuruFocus? Sign up for a free 7-day trial here.
This article first appeared on GuruFocus.

 Yahoo Finanza
Yahoo Finanza 